The future of healthcare
Posted on Apr 1, 2020 by Matthew Gooding
As we stand on the brink of the fourth industrial revolution, healthcare is set to undergo radical changes. Here, we take a look at five Cambridge businesses developing innovative medical technology to change patients’ lives for the better
Across the globe, healthcare systems are coming under increased strain, with a growing and ageing population meaning resources are stretched more thinly than ever before. Technology has long been hailed as the solution to many of the problems facing doctors as they deal with a catalogue of chronic conditions, and now a new generation of entrepreneurs is ready to bring about a healthcare revolution.
Alongside the novel devices and apps that will give patients more control of their own treatment, advances in artificial intelligence and compute power mean analysing big data sets to discover new therapies or pinpoint the most effective treatment is becoming easier than ever before. The med tech market is already pretty hefty, generating revenue of $405bn worldwide in 2018, and many believe this more personalised approach could soon leave the traditional drug companies and their blockbuster drugs on the sidelines.
Cambridge has long been a global centre of excellence when it comes to medtech, with the city’s plethora of design consultancies churning out devices and algorithms to combat a wide range of diseases. Meanwhile, support agencies such as Health Enterprise East and its medtech consultancy are on hand to help entrepreneurs bring their ideas to life.
Bios
A USB port in your body might sound like a gimmicky way of charging your phone on the go, but there’s a serious application for it, which is being developed by the Cambridge start-up, Bios. A specialist in neural engineering, Bios has come up with an interface that allows computers to communicate with the brain. The initial application the company identified
for this was the Prosthetic Interface Device (PID), a universal port, like a USB, designed to allow amputees to connect a range of prostheses directly to their nervous system.
 Bios, a specialist in neural engineering, has come up with an interface that allowscomputers to communicate with the brain
Bios, a specialist in neural engineering, has come up with an interface that allowscomputers to communicate with the brain
Bios hopes the PID will begin clinical trials in the not-so-distant future, but now the company – co-founded by Cambridge graduates Emil Hewage and Oliver Armitage – is expanding its horizons, looking atareas such as neuroceuticals: artificial intelligence-based medical treatments, which adapt signals from the brain to fight disease.

Indeed, earlier this year, Biosreported it has created a neural data biomarker discovery platform capable of quickly and accurately picking out signals from the brain that affect our health. It is hoped this platform can form the basis for developing neuroceuticals, and Bios intends towork with partners in healthcare to explore its potential.
Based at the Future Business Centre, Bios secured a $4.5m seed funding round from investors in the UK, Canada and Silicon Valley last year, which has allowed it to expand its team and open an R&D officein Montreal, a global hotspot for AI talent. Co-founder Emil says: “This funding round marks a new chapter in our company’s history as it gives us the opportunity to leverage our full potential technically and develop our product for the wider ecosystem.
We have an incredible team already made up of experts from a huge range of fields, who have come together to make this incredibly complex technology work seamlessly. Their expertise ranges from machine learning, neuroscience and medical robotics to biotechnology and medical specialists, but we’re looking forward to growing the team even further.
Cambridge Heartwear
Over 15 million people a year globally suffer strokes, and a Cambridge startup hopes to help cut this number with its novel device, which can detect the early signs that all is not well in
the heart. Cambridge Heartwear says its low-cost, next-generation monitor, Heartsense, is the world’s only wireless charged ECG device that is backed by a dynamic artificial intelligence platform. This clever combination assists the doctor in the identification of worrying or dangerous heart rhythms.
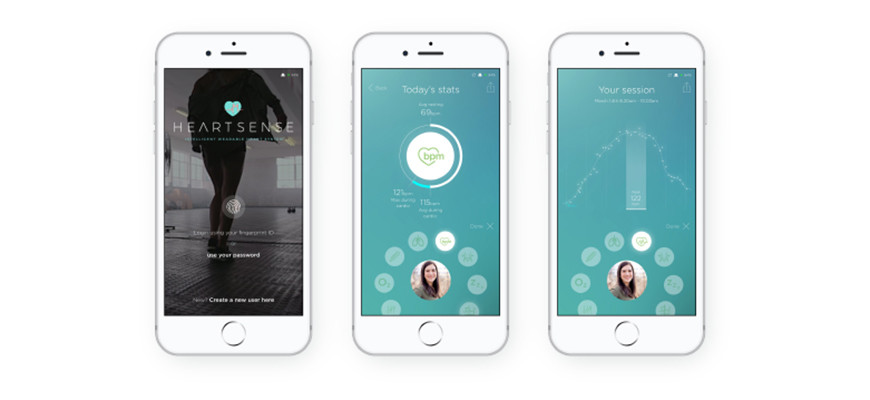
The company’s chairman and co-founder Dr Rameen Shakur says Heartsense is able to detect atrial fibrillation (AF), a palpitation which is often missed, but can often go on to cause a stroke. “It is estimated that 1.4 million people in England have AF, 2.5% of the population,” he says. “This is a significant and growing problem, as that number is set to double by the year 2040, given the obesity, diabetes and hypertension epidemic in the UK and many western countries.
 Atrial fibrillation is a significant and growing problem, and the number of sufferers is set to double by the year 2040
Atrial fibrillation is a significant and growing problem, and the number of sufferers is set to double by the year 2040
“AF is the most common cardiac arrhythmia and an important risk factor for stroke. Treatment with anticoagulants can halve the risk of strokes from AF, but early therapy is important, so identifying and diagnosing AF and other heart rhythms requires a wearable and dynamic device. Heartsense does this.”
Dr Shakur founded the company after seeing first-hand the problems caused by AF during his career as cardiologist and clinical academic. He designed and built Heartsense with the help of co-founders Dr Robert Lowe and Professor Roberto Cipolla, who is a machine

learning expert from Cambridge University’s department of engineering, whose father died from a stroke. Heartsense features an array of sensors that feed data back to an AI platform. This helps identify potentially problematic heart rhythms and flags them up to doctors. A finalist in the Fast Company 2019 World Changing Ideas Awards, Heartsense is currently being trialed in the UK and US.
“We are currently undergoing accreditation for the device and hope to have clinical devices available in early 2020,” Dr Shakur says.
Closed Loop Medicine
Truly personalised medicine is the holy grail for many in healthcare, delivering optimum treatment for patients and welcome efficiencies for care providers. Closed Loop Medicine believes it has come up with a solution that could personalise treatment of many common conditions, combining traditional and digital therapeutics to create what CEO and co-founder Dr Hakim Yadi describes as “software as a medical device”.
 The Closed Loop vision is to put the power of therapy in the hands of the individual, so they can benefit from a truly personalised treatment
The Closed Loop vision is to put the power of therapy in the hands of the individual, so they can benefit from a truly personalised treatment
“The standard doctor/patient interaction is that when you’re sick you go to the doctor, get a prescription, then go back and see them again a few weeks or months later to see if it has worked,” he says. “This means as a patient you’re often left waiting for an appointment, and the doctor has to rely on partial information they received from the patient when making decisions about the efficacy of a treatment. It’s an imperfect feedback loop, and we have a digital means to close that loop.”
Closed Loop’s platform allows closer monitoring of drug and behavioural therapies, and uses these insights to tailor treatments to the individual. Patients receive a customised prescription that combines existing drugs with digital elements and medical devices.
“For example, if we notice a patient is engaging particularly well with behavioural therapy when it involves watching videos, we can ensure they are delivered more video, rather than written, content,” Dr Yadi says.
Closed Loop was founded in 2017, and has secured backing from a number of notable Cambridge investors including Cambridge Angels, IQ Capital and Martlet. Its chairman is renowned biotech entrepreneur and investor Dr Andy Richards, who has been involved in some of the Cambridge cluster’s biggest life science success stories.

Dr Yadi, one of five co-founders of the firm, says the Closed Loop approach could be applied across a wide variety of conditions where treatment involves drug and behavioural elements, and initial areas of focus include sleep disturbance and hypertension. In July it raised £1.3m to fund a research programme looking at a combined drug and digital approach to manage high blood pressure. “No two patients are the same, and our vision is to put the power of therapy in the hands of the individual, so they can benefit from a truly personalised treatment,” he says.
CMR Surgical
Robots will be coming to an operating theatre near you soon, and Cambridge’s CMR Surgical is hoping to grab a big slice of the market. The company is the developer of Versius, a next-generation surgical robot that can perform minimal access, or keyhole, surgery in a range of different areas. Keyhole surgery is far less risky than carrying out an open procedure, and it is hoped the rise of robots such as Versius will help reduce the number of open operations carried out around the world.
Small and portable, meaning it can easily be transferred between operating suites and even different hospitals, CMR believes Versius will also help improve hospital efficiency. The company was founded in 2014, and has grown rapidly in the last five years, closing a record-breaking Series B financing round in 2018. In total the round raised $100m, the largest amount ever secured by a European medical device business. It has since moved into a purpose-built global headquarters at Evolution Business Park near Milton.
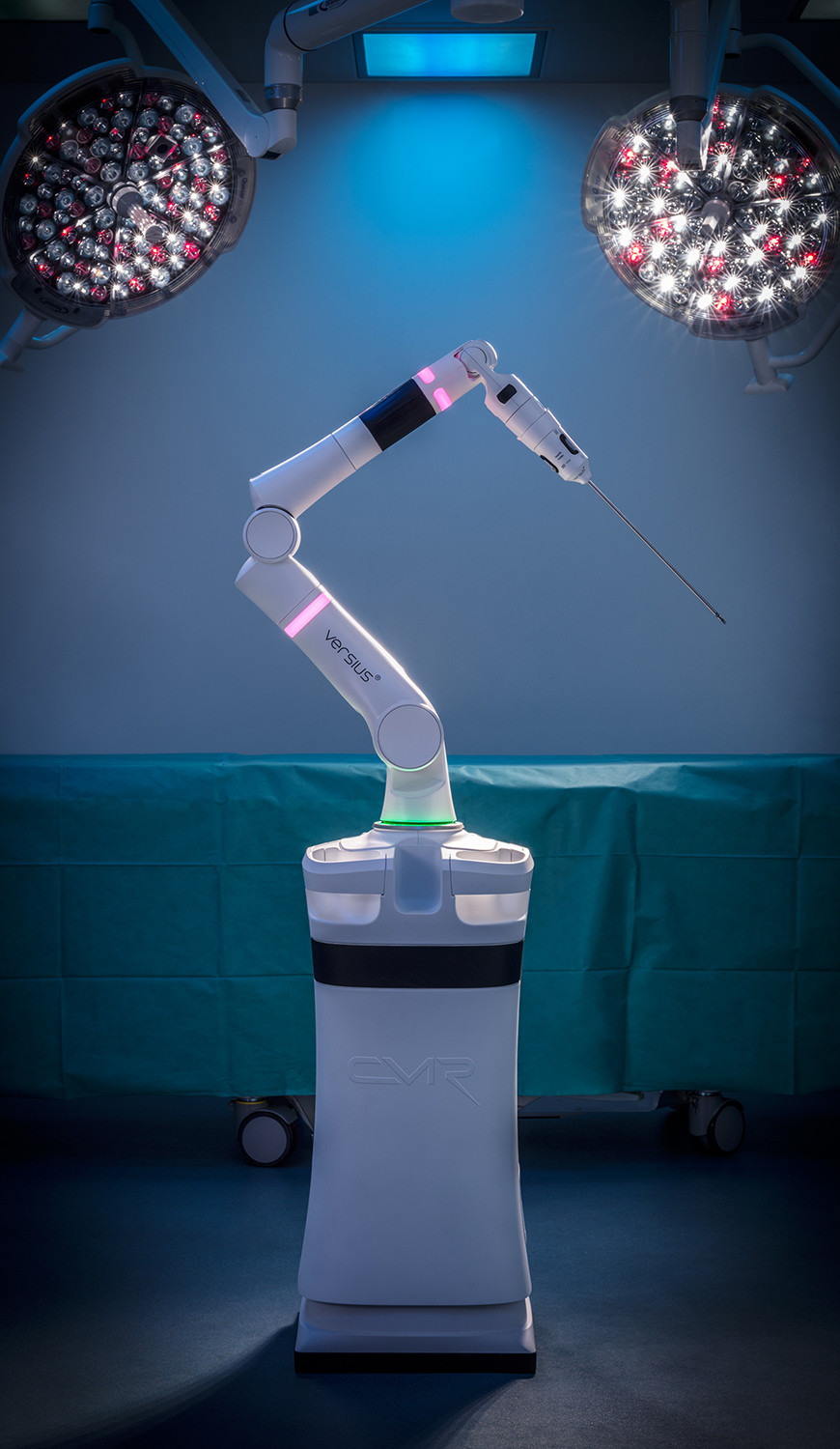
Versius itself received the European CE mark in March, and has already, including carrying out its first operations on human patients. It undertook 30 laparoscopic procedures as part of a clinical trial at Deenanath Mangeshkar Hospital & Research Center in Pune, India. The surgeries consisted of minor, intermediate and major gynaecological and upper gastrointestinal procedures, and no adverse effects were reported as a result of the use of Versius after a 30 day follow-up.
 We look forward to further advancing our mission to bring the benefits of minimal access surgery to everyone who needs it
We look forward to further advancing our mission to bring the benefits of minimal access surgery to everyone who needs it
“This first-in-human series is a significant milestone in bringing Versius to operating theatres around the world,” explains Mark Slack, chief medical officer at CMR Surgical. “These initial results are positive and we look forward to further advancing our mission to bring the benefits of minimal access surgery to everyone who needs it. This series is part of our drive for the responsible introduction of surgical robotic systems that puts safety and effectiveness above all else,” he concludes.
cmrsurgical.com
Owlstone Medical
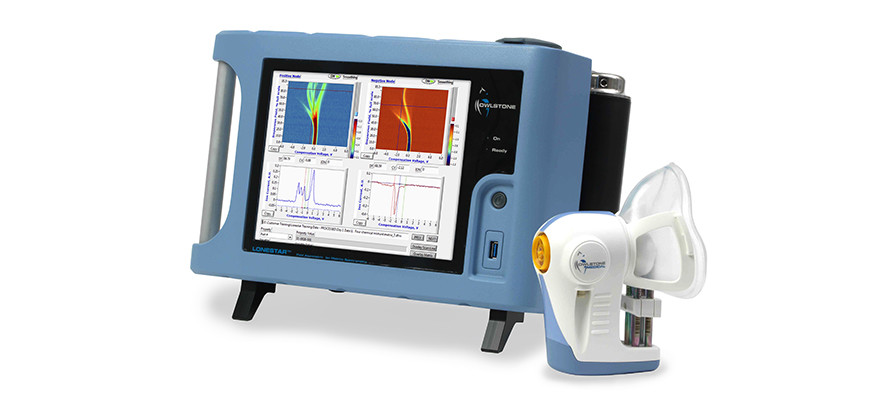
Owlstone is on a mission to save 100,000 lives, and given the company’s, er, breathtaking progress in recent years, few would bet against the Cambridge diagnostic pioneer achieving this noble ambition. Based at the Science Park, Owlstone is the firm behind Breath Biopsy, a platform that operates by detecting and analysing volatile organic compounds (VOCs) found on breath. Changes in these VOCs can indicate the presence of a number of different diseases including cancer, so a simple breath test could quite literally save your life.
“Breath Biopsy is perfectly suited to address two of the major challenges of health care today: early detection and precision medicine,” Owlstone’s director of investor relations, Chris Claxton, tells Cambridge Catalyst.
“For many diseases, the key determining factor in how successful treatment is likely to be is how early in its development the disease is identified. Metabolic changes occur at the very earliest stages of disease, which means the byproducts of this changed metabolism, when volatile, can be detected on breath, potentially well before other physical symptoms have become apparent.
“For precision medicine, because Breath Biopsy directly accesses the underlying molecular mechanisms of disease, it has the potential to provide researchers with critical insights on the onset and development of disease, and to help pharmaceutical companies better understand the mechanism of action of their drugs in development and to help stratify patients and guide decisions on dosing,” Chris explains.
The Breath Biopsy was born out of the personal tragedy that struck Owlstone co-founder and chief executive Billy Boyle in 2012, when his wife Kate died of colon cancer. She wasn’t diagnosed until the disease had reached an advanced stage, and since then Billy and his firm have been dedicated to developing technology to ensure other patients don’t suffer the same fate.
 Breath Biopsy has the potential to provide researcherswith critical insights on the onset and development of disease, and to help pharmaceutical companies
Breath Biopsy has the potential to provide researcherswith critical insights on the onset and development of disease, and to help pharmaceutical companies
Owlstone’s ReCIVA breath sampling device won the MacRobert Award, the top prize in UK engineering, in 2018. No wonder then, that the company has proved popular with investors, raking in over $73m in the last three years.

With its products still in development, Chris says Owlstone Medical has plenty to keep it busy in the near future.
He says: “We are focused on continuing to deliver on all fronts for the business: building deeper ties with current and new pharmaceutical and major academic customers in the UK, US and around the world; driving our clinical trials towards reporting milestones; and developing tests, including for drug metabolism measuring using EVOC probes and environmental exposure monitoring to be launched in the near-term.”



